Xanthan gum, also known as Yellow Adhesive or Xanthomonas Polysaccharide, is a polysaccharide produced through the fermentation of Xanthomonas Campestris. Due to its unique macromolecular structure and colloidal properties, it possesses multiple functions. It serves as an emulsifier, stabilizer, gel thickener, impregnating compound, membrane shaping agent and more. Xanthan gum powder finds extensive application in various sectors of the economy, earning it the title of “the monosodium glutamate of the industry.” As the largest-scale microbial polysaccharide with a wide range of uses, it plays a significant role in multiple industries. Chimique Phytokem is one of the leading Xanthan Gum manufacturer in India.
- +91- 1255-278111
- info@chimiquephytokem.com

XANTHAN GUM
BASIC INFORMATION
Xanthan gum is the ultimate biopolymer for thickening, suspension, emulsification and stabilization. With its unique molecular structure containing pyroracemic acid groups, xanthan gum exhibits exceptional performance. As a long-chain macromolecule with additional functional groups, it showcases distinct properties under specific conditions. It adopts various conformations in aqueous solutions, displaying versatile characteristics depending on the environment.
- Superior Suspension and Emulsifying Properties:
Xanthan gum excels in suspending insoluble solids and stabilizing oil droplets, ensuring optimal suspension effects. - Excellent Water Solubility:
Xanthan gum rapidly dissolves in water, demonstrating exceptional solubility. It can even dissolve in cold water, simplifying processing and enhancing convenience. - Efficient Thickening Agent:
Xanthan gum solutions exhibit low concentration but high viscosity, making it an efficient thickening agent. A 1% aqueous solution of xanthan gum is equivalent to 100 times the viscosity of gelatin. - Remarkable Pseudoplasticity:
Xanthan gum solutions display high viscosity under static or low shear conditions, which sharply decreases under high shear. Its unique molecular structure remains unchanged. Upon eliminating the shearing action, the original viscosity promptly recovers. This exceptional pseudoplasticity effectively stabilizes suspension liquids and emulsions. - Temperature Stability:
Xanthan gum viscosity remains unaffected by temperature changes. While other polysaccharides experience viscosity variations with heating, xanthan gum’s aqueous solution maintains virtually unchanged viscosity from 10 to 80°C. Even in low-concentration solutions within a wide temperature range, it exhibits stable high viscosity. - pH Stability:
Xanthan gum solutions demonstrate excellent stability in acidic and alkaline environments. Its viscosity remains unaffected within the pH range of 5-10, with only slight changes observed below pH 4 or above pH 11. - Salt Stability:
Xanthan gum solutions maintain their viscosity when mixed with various salt solutions, including potassium, sodium, calcium, magnesium, and others. Even under high salt concentrations, it retains solubility, avoiding precipitation and flocculation in saturated salt solutions while experiencing minimal impact on viscosity. - Enzymatic Hydrolysis Stability:
Xanthan gum’s stable double helix structure exhibits exceptional resistance to enzymatic degradation. It withstands enzymes like protease, amylase, cellulose, and hemicellulase, remaining intact and undegraded.
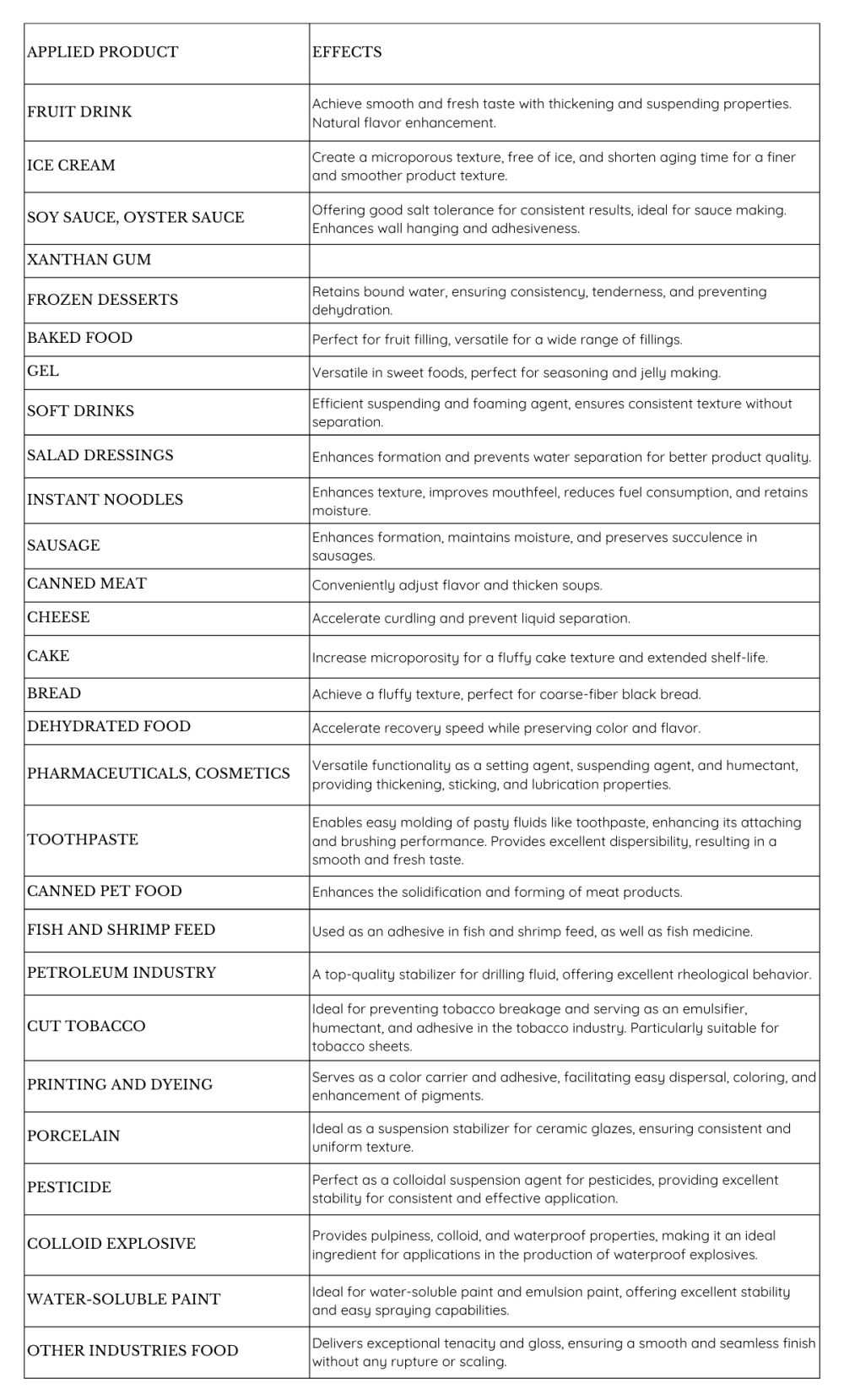
In various industries, xanthan gum serves as a versatile additive, functioning as a stabilizer, thickening agent, and processing assistant. Its applications span a wide range, including canned and bottled foods, bakery products, dairy items, frozen foods, salad dressings, beverages, brewing, confectionery, pastry decorations, and more. Xanthan gum enhances the production process by improving flow, pourability, channeling, and reducing energy consumption.

PLANT NUTRITION FOR A SUSTAINABLE FUTURE
REACH US
Hissar-Rajgarh Road, NH-65,
Village Barwa- Siwani Mandi
127046-Dist. Bhiwani Haryana
info@chimiquephytokem.com
TEL: +91-1255-278111
TEL: +91-8307140067
FAX: +91-1255-278121
Working Hours
Monday – Friday: 7:30am – 18:00pm
Saturday: 7:30am – 1:00pm
Copyrights Chimique Phytokem@2022
